SHORT COMMUNICATION
Molecular detection of Eastern Equine Encephalitis virus in mosquitoes from La Pintada (Antioquia)
Detección molecular del virus de Encefalitis Equina del Este en mosquitos de La Pintada (Antioquia)
Richard Hoyos L,1,2 M.Sc, Juan Suaza V,2 M.Sc, Antonio Tenorio,3 Ph.D, Sandra Uribe,2 Ph.D, Juan Gallego-Gómez,1* Ph.D.
1Universidad de Antioquia, Facultad de Medicina, Grupo de investigación en Medicina Molecular y de Traslación. Carrera 51D Nro. 62-29, Medellín, Colombia.
2Universidad Nacional de Colombia – Sede Medellín, Escuela de Biociencias, Grupo de investigación en Sistemática Molecular. Calle 59A Nro. 63-20, Medellín, Colombia.
3Instituto de Salud - Carlos III. Ctra de Majadahonda-Pozuelo Km 2.2, Madrid, España.
*Correspondence: juanc.gallegomez@gmail.com
Received: September 2014; Accepted: February 2015.
ABSTRACT
Objective. The detection of emerging and re-emerging arboviruses in mosquitoes from urban and rural areas, is fundamental for predict possible epidemic outbreaks in human populations. The Municipality of La Pintada (Antioquia), is characterized by the presence of dry tropical forest relicts, fishing, tourism, farms and mining. An entomological research was performed for explore the possible circulation of arboviruses of public health importance. Materials and methods. Mosquitoes were captured in urban and rural sites in February-April of 2012. The specimens were stored in liquid nitrogens tanks and were grouped using taxonomic keys for genera. RNA extraction from pools and generic/nested RT-PCR was performed for Flavivirus, Alphavirus, Orthobunyavirus (Group Bunyamwera) and Phlebovirus. Results. 1274 mosquitoes were collected, mainly belonging to Culex and Aedes genera. RNA extracts of 64 pools were tested by RT-PCR and one pool was positive for Alphavirus. Sequencing of the RT-PCR product and the analysis with sequences storage in GenBank designate the presence of Eastern equine encephalitis virus (EEEV). Conclusions. This is the first record of natural infection from EEEV in mosquitoes from La Pintada (Antioquia), an area with ecological elements that favor the emergence of emerging and re-emerging arboviruses of medical and veterinarian importance.
Key words: Arboviruses, Culicidae, Eastern Equine Encephalitis virus, Neighbor-joining, RT-PCR (Source: MESH, CAB).
RESUMEN
Objetivo. La detección de arbovirus emergentes y re-emergentes a partir de mosquitos provenientes de áreas semi-urbanas y rurales es fundamental para predecir posibles eventos epidémicos en poblaciones humanas. En el municipio de La Pintada (Departamento de Antioquia), caracterizado por la presencia de remanentes de bosque seco tropical, actividades de pesca, turismo, explotación agropecuaria y minera, se realizó un estudio entomológico para explorar la posible presencia y circulación de arbovirus de importancia en salud pública. Materiales y Métodos. Los mosquitos adultos fueron recolectados en sitios urbanos y rurales en febrero-abril del año 2012. Los especímenes colectados fueron almacenados en tanques de nitrógeno líquido, y luego separados en grupos utilizando claves taxonómicas para género. Para la detección de los virus se realizó extracción de RNA y RT-PCR genérica/anidada para los géneros Flavivirus, Alphavirus, Orthobunyavirus (Grupo Bunyamwera) y Phlebovirus. Resultados. 1274 mosquitos adultos e inmaduros fueron colectados, pertenecientes en su mayoría a los géneros Culex y Aedes. Los extractos de RNA de 64 pools fueron evaluados por RT-PCR. Se encontró un pool positivo para Alphavirus. La secuenciación de los productos de RT-PCR y el análisis con secuencias depositadas en GenBank indica la presencia del virus de Encefalitis Equina del Este (EEEV). Conclusiones. Este es el primer registro de infección natural del virus de encefalitis equina del este (EEEV) en mosquitos de La Pintada (Antioquia, Colombia), un área con factores ecológicos aptos para la emergencia y re-emergencia de arbovirus de importancia veterinaria y médica.
Palabras clave: Arbovirus, Culicidae, EEEV, Neighbor-joining, RT-PCR (Fuentes: MESH, CAB).
INTRODUCTION
Between 1940 and 2004 close to 300 events of emerging diseases were identified in humans around the globe and only in the last 30 years 100 new viruses implicated in new viral diseases have been recognized (1). Most of these considered pathogens introduced in new host populations (emerging), or those that expand quickly in their geographic distribution range, with a corresponding increase of cases and epidemic outbreaks (1,2). In terms of public health, the most important emerging viruses are the arbovirus (arhtropod-borne viruses) transmitted by mosquitoes because they cause viremia in humans, encephalitis and hemorrhagic fevers. These viruses belong to three main families: Togaviridae, Flaviviridae and Bunyaviridae (1,2).
In Colombia, the presence of arbovirus in mosquitoes, vertebrate hosts and infected humans, or with serological contact evidence, has been recorded for Alphavirus (Encephalitis Venezuelan Equine, Encephalitis Equine East, Mayaro), Flavivirus (Dengue, Yellow fever, Virus of the West of the Nile, encephalitis of Saint Louis) and Orthobunyavirus (Guaroa, Wyeomyia) (3-7).
The high diversity of mosquitos of the Haemagogus, Aedes and Culex genera (subgenera Melanoconion and Culex) (3,4,6), the fragmentation of the natural ecosystems and the presence of vertebrae hosts in different areas of the Colombian geography (4) have favored the human-vector-arbovirus contact; increasing the probability of epidemic outbreaks of emerging and re-emerging diseases (3,4,6). In this context, the tracking of viruses in potential mosquito vectors is an important step in the prevention and future control of viral diseases. The objective of this study was to track the natural infection by arbovirus of the Alphavirus, Flavivirus, Phlebovirus and Bunyamwera group genera (Orthobunyavirus) in mosquitos of the municipality of La Pintada by a RT-PCR for each viral genera and a nested RT-PCR for sequencing and identification of the present virus.
MATERIALS AND METHODS
Capture of mosquitoes. The entomological collections were conducted in February and April 2012 in the municipality of La Pintada (Antioquia, Colombia), (geographic coordinates: 5°44’25.63” N, 75°36’20.18” O). The adult mosquitoes were captured using CDC light traps with carbon dioxide baits (CO2) for 13 hours (5: 00PM-6: 00AM) per day, as well as active search using vacuums in rural area forest fragments and in the peridomicile of the urban area. For the case of the immature states (larvae and pupas), the mosquitoes were captured using pipettes together with the water from the breeding grounds that were identified. The captured mosquitoes were transported in liquid nitrogen tanks to the Biology and Systemic lab of the National University of Colombia, Medellin campus. The mosquitoes were grouped in pools of 2-20 individuals according to their external morphologic characteristics to separate genera, place and date of capture.
Molecular detection. Each pool was mechanically marked with a plastic mortar in an Eppendorf tube with RLT buffer and the total RNA was extracted using a commercial kit RNeasy (Qiagen, Valencia). The chain reaction of the polymerase prior to inverse transcription for viral genera (generic RT-PCR), was performed with the One-Step RT-PCR (Qiagen, Valencia) kit, using the RNA extracts that belong to the mosquito pools; and the nested RT-PCR was performed from the positive cDNA for the generic RT-PCR, using the protocols previously described for Alphavirus (8), Flavivirus (9), Phlebovirus (10) and Orthobunyavirus (11).
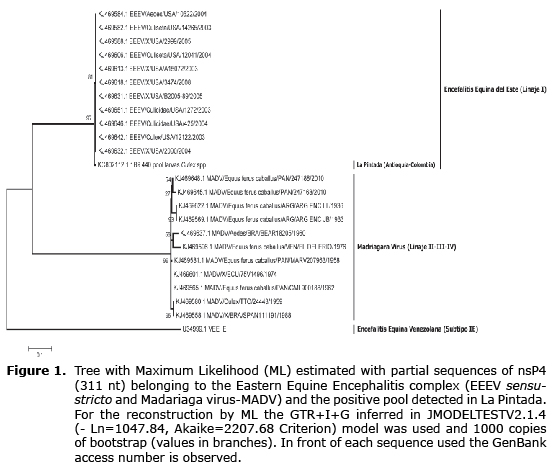
Sequence analysis. The positive PCR products both for the generic and the nested RT-PCR were sequenced following the Sanger en Macrogen (Korea) method. The sequences obtained were edited in BioEditv7.0.5 (http://www.mbio.ncsu.edu/bioedit/bioedit.html) and aligned in CLUSTALW with sequences of the viral group detected available in the Genbank database, prior to their identification by nucleotide similarity using the BLASTN algorithm available in NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Maximum Likelihood (ML) was used for the alignment for the reconstruction of a phylogenetic tree with the PHYMLv6.0 software to identify the identity of the viral sequences detected.
RESULTS
A total of 64 pools formed from 1274 adult mosquitoes were evaluated. 84% corresponded to individuals of the Culex and Aedes genera and in less quantity, the Coquilletidia, Mansonia, Psorophora and Anopheles genera were found. A pool corresponding to the mosquitoes of the Culex genera that was captured in the urban area was positive for the generic RT-PCR and for the nested RT-PCR for the detection of the Alphavirus and the specific viral identification (8). The resulting sequence of 147 nt that originated from the nested RT-PCR allowed for its identification as an Eastern Equine Encephalitis Virus (EEEV), based on the similarity obtained with the BLASTN algorithm (99% of similarity). The virus detected appears included as a complex formed by two species: Eastern Equine Encephalitis sensu stricto and Madariaga virus (MADV). The latter with three lineages that group isolated strains in South America.
To determine specifically the identity of the virus, a sequence obtained from the generic RT-PCR for the Alphavirus was used because it presents greater length and variability (Genbank access number: KC802112), and the sequences available in GenBank for Alphavirus EEEV and MADV. The alignment from the 311 nt fragment of the NSP4 gene (Non-structural Protein 4 - subunit RNA-dependent of polymerase viral RNA) and subsequent phylogenetic reconstruction for Maximum Likelihood identifies the detected virus as EEEV - lineage I (Figure 1), differentiating it from the MADV with high bootstrapp values (>90).
Pools of mosquitoes evaluated were 64, including 1274 individuals (adults and immature), from which a minimum infection rate of 0,784 could be inferred (12). The occurrence of false positives in the molecular detection was excluded by genomic sequence evaluation of all the potential sources of Alphavirus in our lab and the positive control used for these genera was Venezuelan Equine Encephalitis (Rio Negro virus).
DISCUSSION
The molecular detection of the Eastern Equine Encephalitis Virus (EEEV) in this study of emerging arbovirus is the first report for Antioquia of the natural infection in mosquitoes. The epidemiology of EEEV has not been studied in depth and in general, only equine cases are recorded annual since human cases are rare (4,13) with the exception of the plague recorded in the province of the Darien - Panama (14).
The natural infection of mosquitoes with EEEV in South America has been associated with species of the Culex (Melanoconion) - Spissipes Section subgenera like Culex taeniopus, Culex ocossa and Culex pedroi (15), suggesting that it is the main agent for transmission to small mammals and birds in enzootic foci, and in second place the Mansonia spp., and Aedes fulvus (3-4,6); however, the taxonomic determination of the species belonging to the Melanoconion subgenera is not very precise and complex because of its morphological similarity with the species that belong to the group (6,15).
The circulation of EEEV in Colombia was reported for the first time as serologic evidence in two monkeys (“spider monkeys” - Ateles sp, “capuchin monkey” - Cebus sp) in San Vicente del Chucurí (Santander). However, there are prior records of activity in jungle areas of the Magalena Medio region (personal communication from Downs 1961 cited by Groot 1964). Subsequent viral activity has been found in other areas of Colombia like Casanare, Boyaca, Caribbean coast, isolating them from horses and hamsters (4). There are records of an EEEV plague in 1992 that originated 13 fatal cases in equines in Antioquia (4), considering this region a high risk area because of the presence of the virus, competent mosquito species, environmental factors and the presence of areas dedicated to bovine/equine breeding.
The presence of EEEV lineage I sensu stricto in La Pintada - Antioquia, adds to the evidence of the diversity of circulating strains in different geographic areas of Colombia; it also suggests the viral exchange between North and South America. Similar evidence is the isolation of two South American strains of EEEV from migratory birds in Mississippi (USA) (13). However, it is desirable to characterize additional sequences of the virus to precise possible substitution and adaptation events that would possibly generate more biological efficiency in new hosts and as a consequence, affect the virulence, which would impact the ecology of the hosts and vectors that allow for the viral emergence in human populations (14) and therefore, originate clinical-pathogenic symptoms in humans; also, it is necessary to determine the presence of other EEEV linages in La Pintada. Recently, the taxonomy of the EEEV complex has been reviewed through phylogenomic analysis and it has been separated in two species: EEEV sensu stricto (lineage I) and Madariaga virus (MADV). The South American strains are found in the latter, which belong to the II-III-IV linages of the complex (15).
Subsequent entomological works of authors using classic and molecular taxonomy: genetic bar code (cytochrome c oxidase I), have allowed for the identification of mosquito species in the same area of this study, with prior records of natural infection with EEEV and the Venezuelan Equine Encephalitis virus (VEEV), among other arbovirus of medical interest. These species are Culex nigripalpus, Culex quinquefasciatus, Aedes aegypti, Psorophora ferox, Ochlerotattus spp. (Unpublished data).
The detection of low rates of natural infection with EEEV in mosquitos together with the presence of bovine/equine cattle, potential vector species and the possible role of equines in the amplification of the detected virus, allow proposing this area as a risk area for the emergence of this arbovirus. It is important to consider the high evolutionary proximity of the detected virus with North American strains of the virus belonging to lineage I or EEEV sensu stricto. Additionally, it is relevant to perform a detailed inventory of the possible vertebrae reservoirs (birds and small mammals), as well as a serologic/clinical survey to identify the circulation of the virus in humans and to identify the possible cases in equines and humans, which can be non-apparent in their clinical symptoms or confused with other arbovirus that circulate in the area, such as the Dengue virus or the Venezuelan Equine Encefalitis virus.
Acknowledgments
Colciencias, for the scholarship granted to Richard Hoyos Lopez (Grant-528) and the financing of this work under project 111549326198. The School of Medicine of the University of Antioquia, for granting Exclusive Dedication to Juan Carlos Gallego-Gomez. Diego Puerta for support in the captures and the lab.
REFERENCES
1. Jones K. Global trends in emerging infectious diseases. Nat 2008; 451:990-993.
2. Weaver S, Reisen W. Present and Future arboviral threats. Antiviral Res 2010; 85(2):328-345.
3. Groot H, Morales A, Romero M, Ferro C, Prías E, Vidales H, et al. Estudios de arbovirosis en Colombia en la década de 1970. Biomédica 1996; 16:331-344.
4. Mesa FA, Cardenas JA, Villamil LC. Las Encefalitis Equinas en Colombia. Bogotá D.C, Editorial Universidad Nacional de Colombia; 2005.
5. Osorio J, Ciuoderis K, Lopera J, Piedrahita L, Murphy D, Levasseur J et al. Characterization of west nile viruses isolated from captive American Flamingoes (Phoenicopterous ruber) in Medellin, Colombia. Am J Trop Med Hyg 2012; 87(3):565-572.
6. Ferro C, Boshell J, Moncayo A, Gonzalez M, Ahumada M, Kang W, et al. Natural Enzootic Vectors of Venezuelan equine encephalitis virus in the Magdalena Valley, Colombia. Emerg Infect Dis 2003; 9(1):49-54.
7. Mattar S, Komar N, Young G, Alvarez J, Gonzalez M. Seroconversion for West Nile and St. Louis encephalitis viruses among sentinel horses in Colombia. Mem Inst Oswaldo Cruz 2011; 106(8):976-979.
8. Sánchez-Seco M, Rosario D, Quiroz E, Guzmán G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J Virol Methods 2001; 95:153-161.
9. Sánchez-Seco M, Rosario D, Domingo C, Hernández L, Valdés K, Guzmán M, Tenorio A. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods 2005; 126:101-109.
10. Sánchez-Seco M, Echevarría J, Hernández L, Estévez D, Navarro-Mari J, Tenorio A. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J Med Virol 2003; 71(1):140-149.
11. Kuno G, Mitchell C, Chang G, Smith G. Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. J Clin Microbiol 1996; 34(5):1184-1188.
12. Gu W, Unnasch T, Katholi C, Lampman R, Novak R. Fundamental issues in mosquito surveillance for arboviral transmission. Trans R Soc Trop Med Hyg 2008; 102(8):817-822.
13. Calisher C, Maness K, Lord R, Coleman P. Identification of two South American strains of Eastern equine encephalomyelitis virus from migrant birds captured on the Mississippi delta. Am J Epidemiol 1971; 94(2):172-178.
14. Carrera J, Forrester N, Wang E, Vittor A, Haddow A, López-Vergés S et al. Eastern equine encephalitis in Latin America. N Engl J Med 2013; 369(8):732-744.
15. Arrigo N, Paige A, Weaver S. Evolutionary Patterns of Eastern Equine Encephalitis Virus in North versus South America Suggest Ecological Differences and Taxonomic Revision. J Virol 2010; 84(2):1014-1025.